Our Science
Medical Need
Given the impact of alopecia on individuals’ emotional well-being and quality of life, along with the desire for effective treatments, there is a high medical need for research, innovation, and improved treatment options for alopecia. Continued advancements in understanding the underlying causes of alopecia and developing targeted therapies can help address this medical need and improve the lives of those affected by this condition.
Product characteristics and mode of action
Our product, the recombinant expressed soluble CD83 molecule (sCD83), consists of the extracellular domain of the membrane bound form of CD83 (mCD83). We discovered the anti-inflammatory properties of sCD83 and proofed its pro-resolving therapeutic potential in several pre-clinical autoimmune as well as transplantation models. Interestingly, in this context sCD83 application reduced disease symptoms and enhanced allograft survival, not just in a preventive application form but even if applied therapeutically (Grosche et al., Front. Immunol.: 2020; Peckert-Maier et al., Int. J. Mol. Sci.: 2022, Peckert-Maier et al., Am. J. Transplant.: 2022). In a very recent pre-clinical study, we reported for the first time that sCD83 also improves and accelerates wound healing processes in vivo (Royzman et al., Front. Immunol. 2022).
Using transcriptome analyses we identified that also pathways associated with hair growth and hair follicle development were induced in the presence of sCD83. These observations prompted us to investigate this knowledge in further details and discovered that sCD83 accelerated hair regrowth after excessive epilation (see Fig. 1 below).
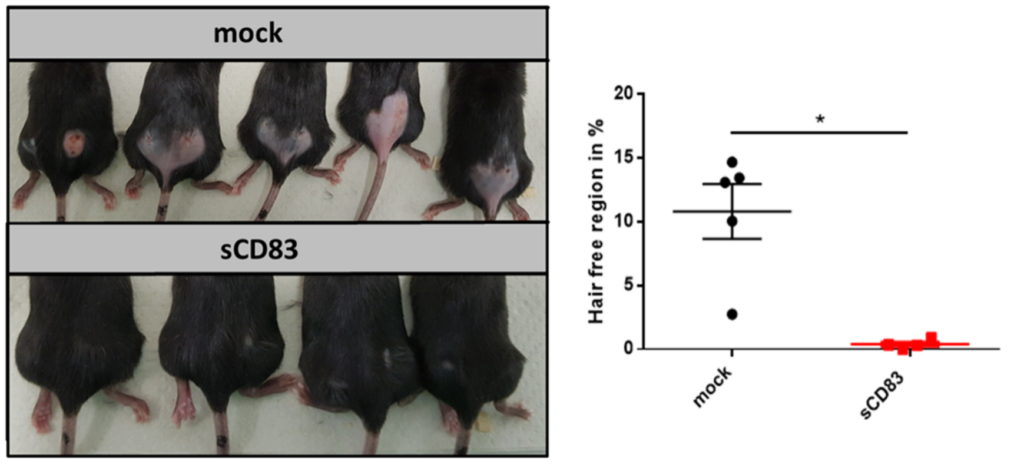
Figure 1: Hair regrowth is boosted in sCD83-treated mice.
In addition, using an additional epilation model, sCD83 treatment increased the number of hair follicles as well as the length of the hair shaft, when compared to mock treated control animals (Fig.2).
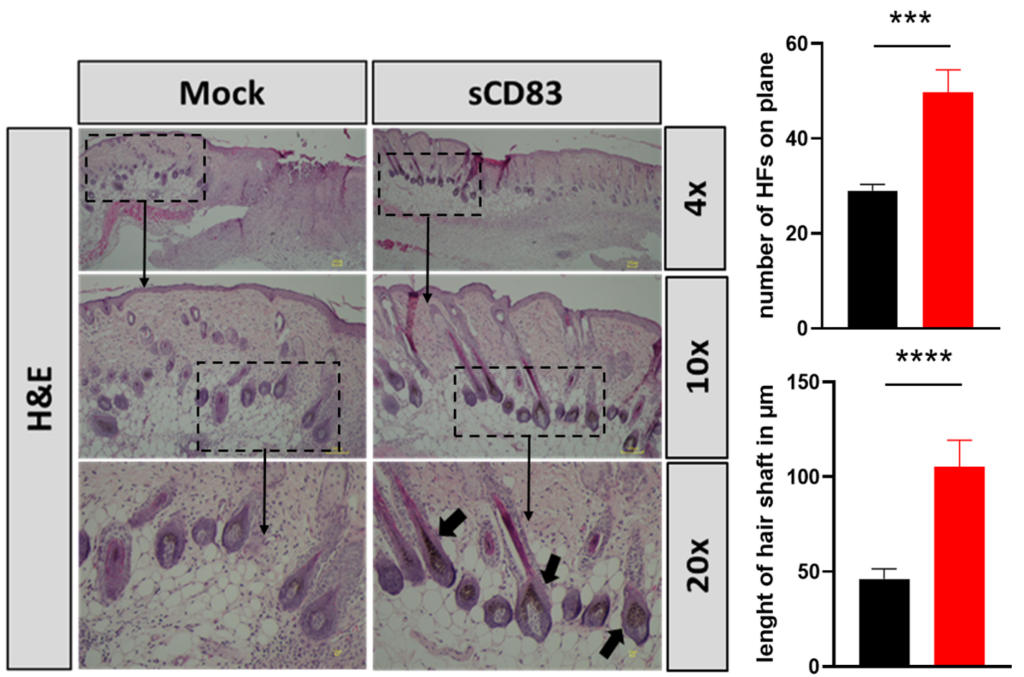
Figure 2: sCD83 boosts new hair follicle formation.
In order to translate our preclinical murine data we performed experiments using human hair follicles and incubated them in vitro with sCD83 or as a control with PBS for 48 and 72 hrs, respectively. As shown in Figure 3, control hair follicles which have been treated with PBS sowed already a progress of the hair cycle towards hair growth arrest (i.e. Catagen-phase), while sCD83 treated hair follicles were still in the growing Anagen-phase. This clearly indicates, that sCD83 also boosts hair growth in a human setting.
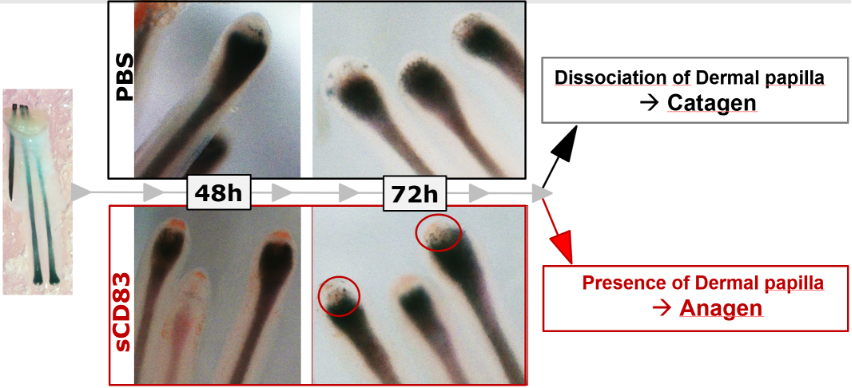
Figure 3: sCD83 prolongs the Anagen = growing phase also in human hair follicles.
Next, we investigated if local administration of sCD83 modulates the number of eyelashes. Therefore, sCD83 was topically administered in the form of eye drops. Surprisingly and noteworthy, the sCD83-treated group revealed increased numbers of eyelashes with a longer and thicker shape, when compared with mock-controls (Fig. 4).

Figure 4: Topical sCD83 application increases the number and length of eyelashes after topical application
In order to get further insights regarding the underlying mechanisms of sCD83 induced hair regrowth, we have analysed the presence of regulatory T cells (Tregs) in dorsal skin biopsies, after epilation. As reported by the group of Rosenblum, the presence of Tregs within the sites of hair follicles in the skin, is associated with enhanced hair growth. In sharp contrast, the ablation of Tregs resulted in impaired Anagen induction and hair regrowth (Ali, N. et al., Cell: 2017). Since we have shown, that sCD83 induces Tregs (Bock, F. et al., J. Immunol.: 2013), we hypothesized that sCD83 increases the numbers of Tregs in treated animals and that this would lead to a better hair growth. Indeed, analyses of skin samples from sCD83 treated animals revealed an accumulation of Tregs in sCD83-treated mice, when compared to the mock-group.
It is known, that sCD83 bind to the cell surface expressed TLR4-MD2 complex and induces an anti-inflammatory signal (Horvatinovich et al., J. Immunol.: 2017; Royzman et al., Front. Immunol.: 2022). Therefore, we analysed if sCD83 also binds to follicular stem cells in order to induce and promote hair growth. Using a new biosensor technique, we were able to show the direct binding of sCD83 to human follicular stem cells.
Taken together, the above described independent experiments clearly support the effect of sCD83 on hair growth.
In this context, sCD83 has a dual mode of action:
- On the one hand sCD83 induces an anti-inflammatory micro-milieu, via the propagation of Tregs which interact with follicular stem cells and thereby activate hair growth.
- Secondly, sCD83 directly binds to follicular stem cells and induces the formation of new hair follicles.
Thus, due to this unique mode of action sCD83 represents a promising treatment strategy for both, the hormone induced androgenic alopecia as well as the autoimmune-mediated alopecia areata (see schematic representation below).
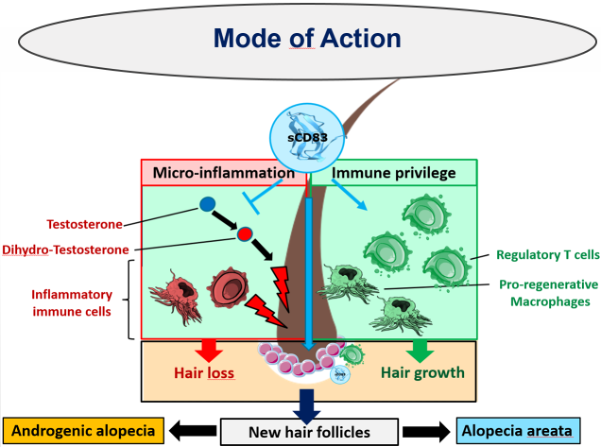
In comparison to current treatments, we aim to induce a long-term hair growth via a direct interaction of our product sCD83 with hair follicle stem cells, which induces the activation and formation of new hair follicles. This has not been achieved by any of the currently approved medications. In addition, sCD83 induces pro-regenerative immune cells i.e. tissue repair macrophages and Tregs, which subsequently activate hair follicle stem cells and thus new hair growth.
Non-Clinical
Hair growth in mice and men relies on several conserved mechanisms between these two species and therefore murine models represents suitable options to investigate the potential of new products to prevent hair loss. In order to show the sCD83-mediated effects on hair growth, we made use of several mouse models (mentioned below). In addition, we already transferred our analyses into the human system, using human derived hair follicles for our ex vivo studies (see below).
- First pre-clinical in vivo data have been obtained using a murine epilation-induced hair growth model. In this model, hair growth is characterised by a strong pigmentation and thickening of the epilated murine skin, up to eight days. Interestingly, sCD83-treated mice showed an accelerated skin pigmentation, indicative for an accelerated onset of the anagen (= hair growth) phase. Histological analyses of sCD83 treated mice revealed a significantly increased number of new hair follicles, which in addition were bigger size and had enlarged shaft length (both statistically significant), when compared to control treated mice. Furthermore, transcriptome analyses showed that sCD83 induces specific transcripts, which are absolutly vital for the induction of new hair growth. In addition, we have shown that sCD83 potently induces so called tissue repair macrophages and regulatory T cells (Tregs). Both are specific immune cells that play a crucial role in hair growth, amongst others via the activation/ induction of new hair follicles.
- Hormone induced androgenic alopecia
In respect to the pathogenesis of the hormone induced androgenic alopecia, the testosterone breakdown product, dihydrotestosterone (DHT) induces a micro inflammation, leading to the breakdown of the immune privilege of the hair follicle and subsequently to hair growth arrest and finally to hair loss. - Preclinical model for androgenetic hair loss:
Using a preclinical murine model for the DHT-induced androgenetic hair loss we revealed, that sCD83 treatment restored hair growth in these mice. This shows, that sCD83 induces new hair growth also in a model, relevant for androgenetic hair loss in humans. - How does sCD83 interfere with androgenetic hair loss associated pathogenic mechanisms?
- sCD83 blocks inflammation and restores the immune privilege of the hair follicle
- sCD83-induces regulatory T cells which directly interact with dermal papillar stem cells, thereby stimulating new hair growth
- sCD83 directly binds to the TLR4-MD2 receptor complex, which is expressed by dermal papillar stem cells, thereby directly promoting new hair growth.
- Autoimmune induced alopecia areata
In contrast to the hormone induced androgenetic hair loss, the second most common form of hair loss, the alopecia areata, is an autoimmune mediated hair loss disorder. Since we have previously shown, that sCD83 strongly prevents autoimmune disorders in several preclinical autoimmune models in vivo, including those for MS, RA and IBD, sCD83 represents an ideal candidate for the treatment of the autoimmune mediated alopecia areata.
- Translation into the human system:
Effects of sCD83 on healthy human-derived hair follicles:
Healthy human hair follicles have been isolated from the back side of the head and incubated with sCD83 for up to 72 hours and analysed by in depth transcriptome sequencing analyses (RNA sequencing). Obtained key findings are:
- The anagen (growing) phase has been prolonged in sCD83-treated human hair follicles
- sCD83 induces hair growth associated pathways, which are important for hair growth and regeneration.
- Upon sCD83-treatment, within the hair follicles a unique stem cell population, representing the source for hair formation, has been highly expanded.
- Effects of sCD83 on hair follicles derived from androgenic alopecia patients:
Hair follicles derived from androgenic alopecia patients are typically miniaturized and only slightly pigmented. Their environment is dominated by molecules, which are associated with DHT-induced androgenic alopecia and impaired stem cell activities. Obtained highlights are:
- When hair follicles derived from androgenic alopecia patients have been incubated with sCD83, hair loss inducing molecules have been reduced
- While transcripts pivotal for hair growth have been strongly induced in sCD83 treated samples.
Thus, these data strongly support our strategy to develop sCD83 as a new treatment option for patients suffering from androgenetic hair loss.
IP
- Solid IP (EP20169899.0 and PCT/EP2021/05) situation and long patent life
- FTO analysis available